[2008-09-10] Cocaine analog in two steps from native plant material
And now a brief sojourn into the world of clandestine chemistry, and the corresponding disclaimers: I have never done this. I will never do this. I don't think anyone should ever do this, because it is illegal and will get you in lots and lots of trouble. Drugs are bad, mmmmkay?
So why post it? Lots of reasons. Among others, these include: 1) As an act of protest against an out-of-control national drug policy, 2) as an exercise in the free expression of ideas, and 3) as a way to get some kind of intellectual satisfaction out of a research project that I am proscribed by law from actually carrying out.
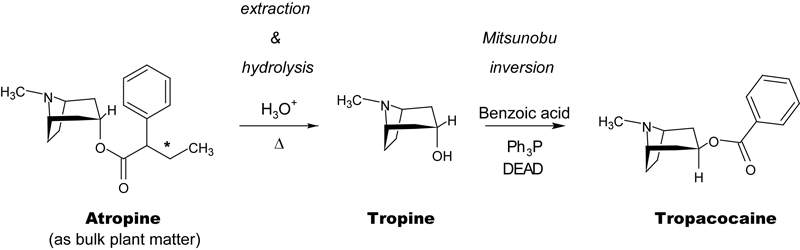
I am certainly not the first to recognize that tropacocaine, which is an analog of cocaine with about 1/10th the potency thereof, is relatively easy to prepare from atropine, which in turn is readily extracted from any number of plant species that grow wild and/or can be legally cultivated in the US. Pseudonymous head-shop author Otto Snow has published a book called THC & Tropacocaine, which is a mish-mash of reprints from various scientific publications arranged so as to allow the astute reader to fill in the blanks, but without making the route explicit and thereby botching up Snow's plausible deniability. He claims, after all, that the book is intended as "a tool for the legal, medical, scientific and political professions and should not be misconstrued as a 'cookbook.'" This is a classic rhetorical Judo-throw in the underground literature: I'm not teaching criminals how to break the law, I'm teaching cops how to catch criminals when they break the law. The first amendment's a wonderful thing, ain't it?
Anyway. While the route itself is valid, the chemistry that Snow very carefully avoids proposing is archaic: His most modern source is from 1923, and if one were to use only the procedures described in his book to go from, say, dried belladonna plants to tropacocaine, no fewer than six steps would be required, counting extraction of starting material from the bulk plant matter. Most of these involve mucking around with the tropine C3 stereocenter to yield the correct "up" isomer on esterification with benzoic anhydride.
Organic chemistry has come a long way since 1923, however, and all I've really done here is apply a modest understanding of post-WWII synthesis to the problem: the Mitsunobu reaction, discovered by Oyo Mitsunobu in 1967, actually allows for stereospecific inversion of an alcohol with an appropriate nucleophile, and is a perfect conceptual fit in this case. There are, moreover, several literature reports of successful Mitsunobu inversion of the C3 alcohol of tropine (see, e.g. Tet. Lett., 42 (2001), 1975-1977.)
Of course, there are some hitches. The Mitsunobu reaction needs to be dry, for instance, which can create considerable inconvenience even for a properly-equipped professional chemist. Also the necessary diethylazodicarboxylate (DEAD) doesn't exactly grow on trees, and is required in stoichiometric amounts. Of course, Bruce Lipshutz's di[4-chlorobenzyl] azodicarboxylate [DCAD] reagent, which is easily recovered and regenerated when the inversion is complete, might be substituted for DEAD to reduce this problem. Likewise, it's worthwhile to note that substitution of 4-fluorobenzoic acid for benzoic acid in the inversion step would yield 4-fluorotropacocaine, which is known to be a considerably more potent cocaine analog than simple tropacocaine.
last modified 2008-09-10